Sometimes the obvious answer is the correct one. M Womersleys were sent a sample of material that had leached out of some restoration work

Sometimes the obvious answer is the correct one. M Womersleys were sent a sample of material that had leached out of some restoration work, from a church at Portglenone, in an area where copings were bedded on lead, and we were asked to determine its make up.
After it was dissolved in distilled water it was tested for different salts and lead. Afterwards part of it was dissolved completely in hydrochloric acid, and part in Hexafluoro silicic acid where it participated lead fluoride.
It was found to be rich in lead oxides and carbonates, without the presence of other salts. Simply, it appears that the deposits consist of lead oxide, lead carbonate and calcium carbonate, which has come from calcium hydroxide washed out of the lime mortar. The mortar was not likely to have been bound by enough of a hydraulic set or carbonated enough, in the first few months of its life, before severe weather hit the mortar.

There are problems which emerge from the use of hot lime and very weakly bound mortars in exposed situations, just as problems emerge for the fabric, when too strong a mix, is used next to weak materials. It is essential to choose the most appropriate mortar for the job and not follow current fashion, which often advocates the sole use of hot, or feebly hydraulic, lime mortar
Related Articles

The steps members of the Waterton’s Wall restoration team, with support from Mark Womersley, have been following to consolidate, conserve and repair this historic wall that represents the successful efforts of Charles Waterton to preserve the wildlife that lived on his estate near Wakefield in West Yorkshire.
1. Fill deep voids behind the wall’s facing stones with deep pointing work. The works involve …
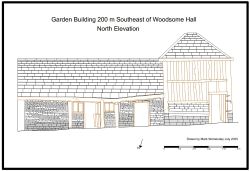
Mark spent a day recording a historic timber-framed garden building at Woodsome Hall
Mark Womersley, as part of his voluntary work with the Yorkshire Vernacular Buildings Study Group, spent…

M Womersleys were delighted to offer a day of tutoring to those who attended the Wentworth Woodhouse Working Party
M Womersleys were delighted to offer a day of tutoring to those who attended the Wentworth Woodhouse…
